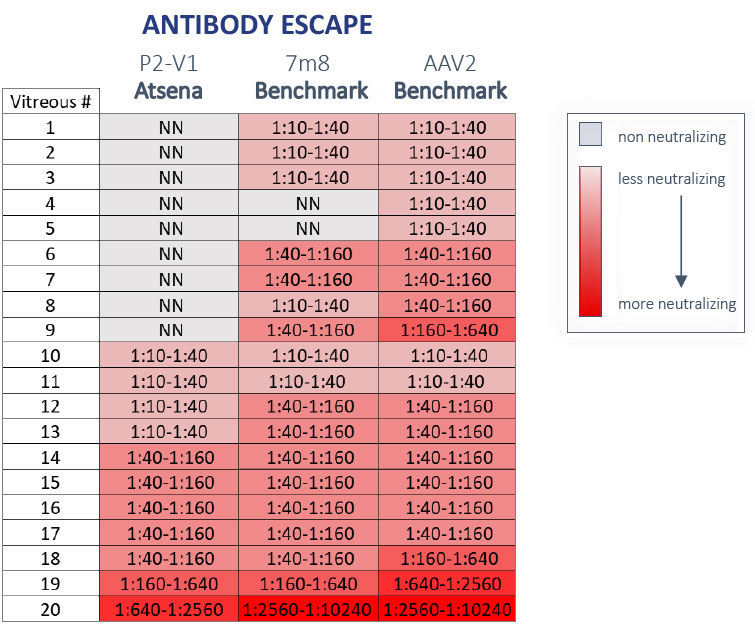
Atsena’s novel AAV capsids for intravitreal injection
have improved potency and the ability to evade pre-existing neutralizing antibodies
Because a significant number of people have been naturally exposed to them, patients often have pre-existing immunity to common AAVs that may prevent them from being candidates for trials or responding to therapy. This is especially important for intravitreally delivered gene therapies. Atsena’s novel AAV capsids for intravitreal injection are engineered to improve potency and selected to avoid detection by the patient’s immune system, expanding the potential patient population and use of therapy.